Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests.
Rapid diagnostic assessments (RDTs) play an important function in the administration and management of malaria an infection.
The histidine-rich protein 2 (PfHRP-2) based mostly RDTs are the mostly used RDTs for malaria analysis in Sudan. Deletion of pfhrp2 in Plasmodium falciparum genome have an effect on the accuracy of PfHRP-2 based mostly RDT kits.
This examine aimed to establish molecular variation of pfhrp2 amongst suspected malaria sufferers from completely different clinics in Omdurman, Sudan.
A noticeable variation between the RDT (Alltest Biotech, China) and nPCR outcomes was noticed, for RDT 78% (46/59) had been P. falciparum constructive, 6.8% (4/59) had been co-infected with each P. falciparum and Plasmodium vivax, 15.3% (9/59) had been detrimental by the RDT.
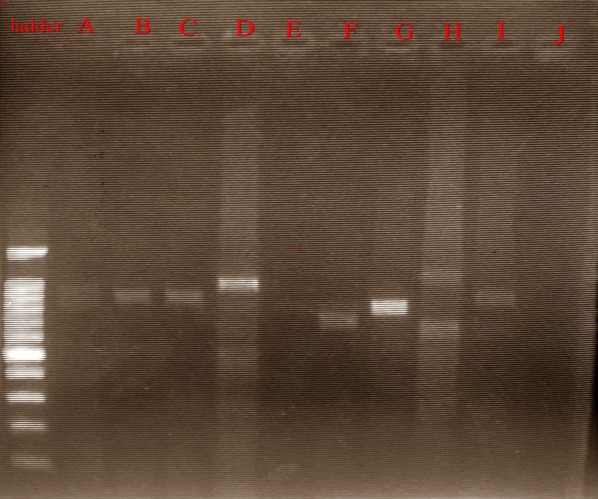
However, when the nPCR was utilized solely 44.1% (26/59) and 55.9% (33/59) was P. falciparum constructive and detrimental respectively. The pfhrp2 was additional amplified kind all nPCR constructive samples.
Only 17 DNA samples had been constructive from the 26 constructive P. falciparum, apparently, variation in band sizes was noticed and additional confirmed by DNA sequencing, and sequencing evaluation revealed a high-level of genetic diversity of the pfhrp2 gene in the parasite inhabitants from the examine space.
However, regardless of excessive sequence variation, diversity of PfHRP2 doesn’t seem to have an effect on RDT performance.

Evaluation of Three Rapid Screening Tests for Detection of Hepatitis C Antibodies on Mass Scale.
Hepatitis C virus (HCV) is a number one well being downside throughout the globe. Only 20% of HCV constructive people know their constructive illness standing. Effective HCV screening assessments are required to display screen each normal and high-risk populations and establish the silent circumstances of HCV.
In this examine, we analyzed the performance of three rapid HCV screening kits. A complete of 300 topics from three populations teams, had been enrolled from Rawalpindi and Islamabad cities of Pakistan. The three teams had been blood donors (n = 50), pregnant girls (n = 50), and hepatitis C constructive people (200).
Blood samples of all the people had been screened on three rapid screening assessments for anti-HCV: CTK Biotech’s OnSite HCV Ab Rapid Test, SD Bioline One Step anti-HCV check, and Intec Products Advanced Quality Rapid Anti-HCV Test. The performance of these three rapid assessments was additionally in contrast with the Roche Anti-HCV II check carried out on the cobas 601 platform based mostly on the electrochemiluminescence immunoassay precept.
In whole, 300 samples had been analyzed on this examine, out of which 208 had been constructive for anti-HCV constructive and 92 had been detrimental for anti-HCV. The sensitivities of the Intec product, SD Bioline, and CTK Biotech had been 98.56%, 97.59%, and 95.67%, respectively. The specificity of SD Bioline and CTK Biotech had been 100%, whereas Intec merchandise confirmed 98.91% specificity.
The constructive predictive worth (PPV) of SD Bioline and CTK Biotech was 100%, however Intec merchandise confirmed 99.51% PPV. The detrimental predictive values of the Intec product, SD Bioline, and CTK Biotech had been 96.80%, 94.84%, and 91.09%, respectively.
There is a dire want to hurry up HCV screening to attain the targets in the World Health Organization international viral hepatitis technique (2016-2021). The rapid assessments evaluated on this examine can be utilized in hepatitis screening on a lot bigger scales.
